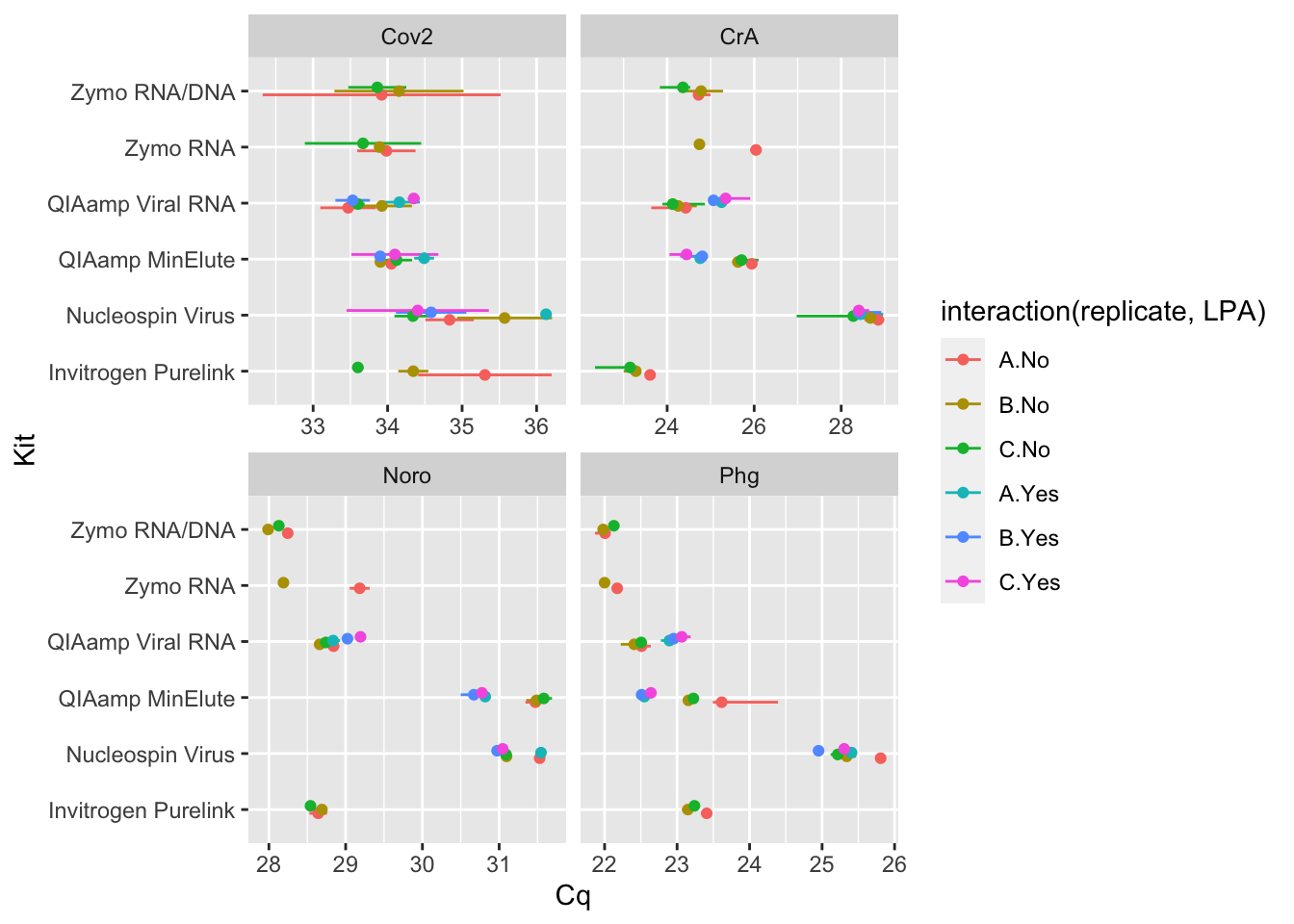
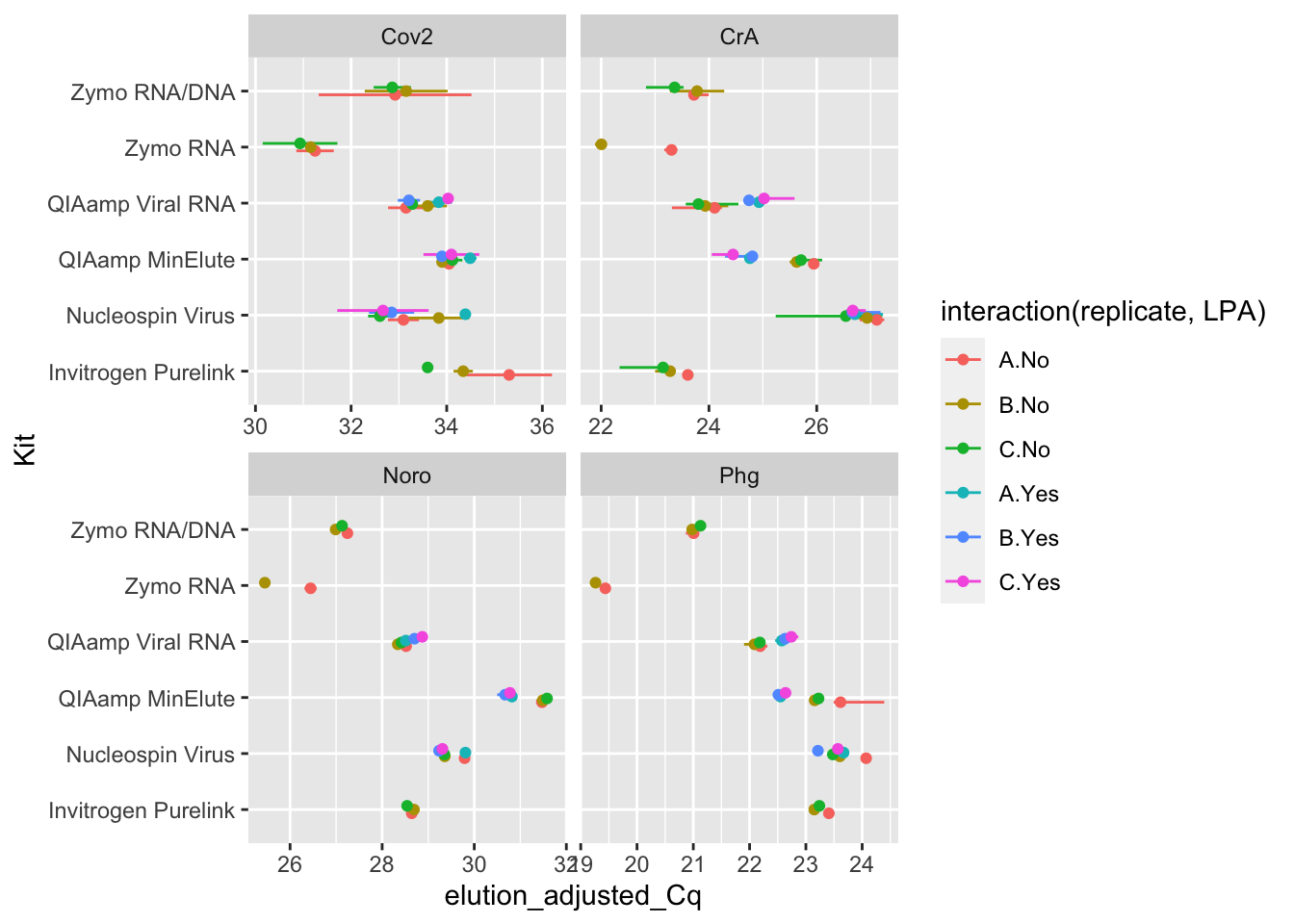
Rows: 333
Columns: 30
$ Well <dbl> 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14,…
$ `Well Position` <chr> "A1", "A2", "A3", "A4", "A5", "A6", "A7", "A8"…
$ Omit <lgl> FALSE, FALSE, FALSE, FALSE, FALSE, FALSE, FALS…
$ Sample <chr> "1_ZR_A", "1_ZR_B", "1_ZR_C", "2_ZDR_A", "2_ZD…
$ Target <chr> "Cov2", "Cov2", "Cov2", "Cov2", "Cov2", "Cov2"…
$ Task <chr> "UNKNOWN", "UNKNOWN", "UNKNOWN", "UNKNOWN", "U…
$ Reporter <chr> "FAM", "FAM", "FAM", "FAM", "FAM", "FAM", "FAM…
$ Quencher <chr> "NFQ-MGB", "NFQ-MGB", "NFQ-MGB", "NFQ-MGB", "N…
$ `Amp Status` <chr> "Amp", "Amp", "Amp", "Amp", "Amp", "Amp", "Amp…
$ `Amp Score` <dbl> 1.1919015, 1.2241620, 1.3026766, 1.3064751, 1.…
$ `Curve Quality` <lgl> NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA…
$ `Result Quality Issues` <lgl> NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA…
$ Cq <dbl> 33.59224, 33.85453, 32.88852, 32.32325, 35.019…
$ `Cq Confidence` <dbl> 0.9630252, 0.9623811, 0.9919308, 0.9907208, 0.…
$ `Cq Mean` <dbl> 33.98352, 33.89186, 33.66943, 33.92028, 34.152…
$ `Cq SD` <dbl> 0.55335254, 0.05279505, 1.10437933, 2.25853860…
$ `Auto Threshold` <lgl> TRUE, TRUE, TRUE, TRUE, TRUE, TRUE, TRUE, TRUE…
$ Threshold <dbl> 0.04098764, 0.04098764, 0.04098764, 0.04098764…
$ `Auto Baseline` <lgl> TRUE, TRUE, TRUE, TRUE, TRUE, TRUE, TRUE, TRUE…
$ `Baseline Start` <dbl> 3, 3, 3, 3, 3, 3, 3, 3, 3, 3, 3, 3, 3, 3, 3, 3…
$ `Baseline End` <dbl> 31, 30, 30, 29, 32, 31, 31, 31, 30, 32, 33, 31…
$ replicate <chr> "A", "B", "C", "A", "B", "C", "A", "B", "C", "…
$ Kit <chr> "Zymo RNA", "Zymo RNA", "Zymo RNA", "Zymo RNA/…
$ treatment_group <chr> "ZQ-RNA", "ZQ-RNA", "ZQ-RNA", "ZQ-RNADNA", "ZQ…
$ LPA <chr> "No", "No", "No", "No", "No", "No", "No", "No"…
$ group <dbl> 3, 3, 3, 3, 3, 3, 3, 3, 3, 1, 1, 1, 3, 3, 3, 3…
$ other_treatment <chr> "Shield", "Shield", "Shield", "Shield", "Shiel…
$ Virus_specific <chr> "Yes", "Yes", "Yes", "Yes", "Yes", "Yes", "No"…
$ FP_Data <chr> "No", "Yes", "No", "No", "Yes", "No", "No", "Y…
$ Elution_volume <dbl> 15, 15, 15, 50, 50, 50, 100, 100, 100, 30, 30,…